From Organoids to Gastruloids
Last year was hailed as 'the year of the organoid' as news of mini-brains, livers and intestines grown in the laboratory hit the headlines. Here, Dr David Turner explains the latest in vitro systems for modelling human development and disease, including embryonic stem cells, organoids and gastruloids
The Biologist 64(5) p14-19
Some of the most important questions in biology relate to how we are formed and develop. For example, how is it that a single fertilised egg is able, in a relatively short space of time, to produce so many different cell types, tissues and organs, and position them so precisely within the body? Why does human development occasionally go wrong, producing birth defects and pathological conditions?
The answers to these questions are vital in uncovering the mechanisms behind disease states, eventually instructing us in ways to treat them through regenerative medicine, or manage their severity through better medicines and pharmaceuticals.
We can use a number of experimental tools and techniques to tease apart some of these questions, traditionally using genetic experiments in embryos from animals such as the fruit fly, frog, chicken, zebrafish and mouse. In terms of mammalian development, the mouse has proven exceptionally useful as a model system.
Although many developmental processes and patterning events are conserved throughout diverse animal species, how sure are we that what we see in the mouse (and other model systems) recapitulates human development?
Mice and other model systems have a number of limitations. For example: it is not always possible to discount the effects of redundancy between genes when assessing the effect of mutant phenotypes (i.e. biological systems are buffered, and multiple genes may have similar functions); mechanical forces, which have been shown to play an important role in development, are very difficult to assess; there is a great deal of expense involved in maintaining mouse lines (and generating new ones with specific traits), and it is difficult and technically challenging to experimentally manipulate the mouse embryo at the early stages.
There are also ethical considerations to take into account when using mice and their embryos (although a number of initiatives are underway to dramatically reduce or replace the numbers of mice and other animals involved in experiments and refine their use, such as the National Centre for the Replacement, Refinement and Reduction of Animals in Research, NC3Rs).
What is needed is a fully tractable system (one that's easy to control experimentally) that is cheaper than in vivo work, is ethically responsible, and can be utilised to ask specific questions regarding developmental processes and differentiation, which can eventually be targeted to understanding human development and disease.
Stem cells, disease and development
Embryonic stem cells (ESCs) offer an alternative and, arguably, parallel route to dissecting the development of embryos, delineating the processes and mechanisms utilised physiologically. ESCs are a self-renewing, pluripotent population of cells derived from the early mouse embryo which can give rise to all the tissues and organs of the embryo proper (they were first isolated from mouse blastocysts by Evans and Kaufmann in the 1980s).
The pluripotent, self-renewing trait of ESCs is essential during development and useful experimentally, as it prevents premature exhaustion of the cells as they are cycled into different cell fates in vivo, and enables us to culture them indefinitely in the laboratory.
Careful experimentation has determined many of the genes and signals involved in patterning the early embryo, and it is this well-grounded understanding that enables us to guide ESCs towards specific tissue types and cell fates by applying signals or modifying cells' gene expression in culture. For example, neural tissues can be generated by applying retinoic acid or inhibiting bone morphogenetic protein (BMP) signalling, and beating cardiomyocytes can be enriched through application of BMP, Activin A and vascular endothelial growth factor.
It is often the case that by using a tissue culture-based approach, new insights can be gained regarding the signals involved in cell fate specification. Examples of this include our recent work on the role of Wnt/ β-Catenin and fibroblast growth factor signalling in generating a population of cells that serves as a pool for generating the body axis[1] or how cells resolve binary fate decisions depending on the signalling environment they are exposed to and the time in which they see these signals[2].
ESCs are, when compared with in vivo studies in the mouse, exceptionally easy to manipulate (genetically and chemically), reduce the necessity for animal experimentation and are orders of magnitude less expensive than keeping mice in the laboratory. However, there are a few disadvantages that have the potential to compromise our complete reliance on ESCs with respect to in vivo work.
One of these involves the topographical differences between a 2D layer of cells grown on a plate and the 3D nature of a whole embryo – the 3D spatial organisation in the embryo between cells and tissues is not replicated on a tissue culture plate.
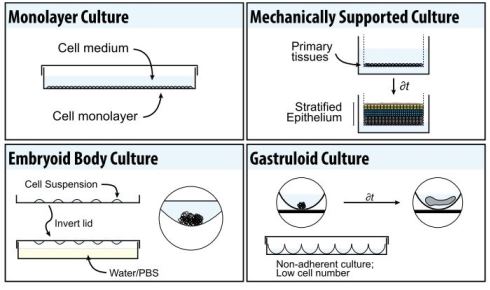
This limitation can to some extent be solved by culturing cells as 3D aggregates known as embryoid bodies (Fig. 1). To generate these bodies, cells are typically plated in little droplets on the inside lid of a tissue culture dish and inverted so they form aggregates at the bottom of a droplet through gravity (Fig. 1). Cells grown in this way produce many cell types associated with the three embryonic germ layers over time, and can even form spontaneously beating regions as cardiac precursors are generated.
Unfortunately, embryoid bodies are highly disorganised[3,4] and fail to produce structures with any similarity to the embryo. Their usefulness is therefore limited to broad questions on the signals required for differentiation of various cell types, as well as generating precursor populations for further differentiation protocols.
The organoid revolution
Early studies using 3D structures, in parallel with work on embryoid bodies, used artificial scaffolds and matrices to provide support for growing tissues in what is known as mechanically supported air-liquid interface culture. When skin or oesophageal primary keratinocytes are grown in this way (Fig. 1), they spontaneously differentiate and form self-organised, stratified tissues. However, within the last decade, there has been something of a revolution in what can be accomplished in this field, with the rise of organoids (Fig. 2).
Generally speaking, cellular material (such as mouse or human ESCs, tissue fragments, primary cells and so on) that is grown in 3D, and over time, can form structures very similar in the patterning and, sometimes, function to their in vivo counterpart[5] following the same developmental progression as in the embryo. Brain (mini-brains), optic cup and gut organoids have been produced, to name just a few (see Fig. 2).
These have the very real potential to be powerful model systems for probing both how organs develop normally, and how pathologies and disease states can affect their development.
One of the most important examples of this came from the Hongjun Song and Guo-li Ming's research groups and modelled the effect of the Zika virus on human brain development[6] (Fig. 2). The Zika virus, declared by the World Health Organization as a public health emergency of international concern, is particularly problematic for pregnant women, as it is passed on to the developing foetus, resulting in developmental defects and neurological disorders such as microcephaly.
By generating brain organoids, which mimic key aspects of human cortical development, from human induced pluripotency stem cells (iPSCs; cells from adult organisms that have been essentially reprogrammed to their embryonic state) and exposing them to the Zika virus, they were able to determine the neural cells the virus preferentially targeted and how microcephaly may be established[6] (Fig. 2).
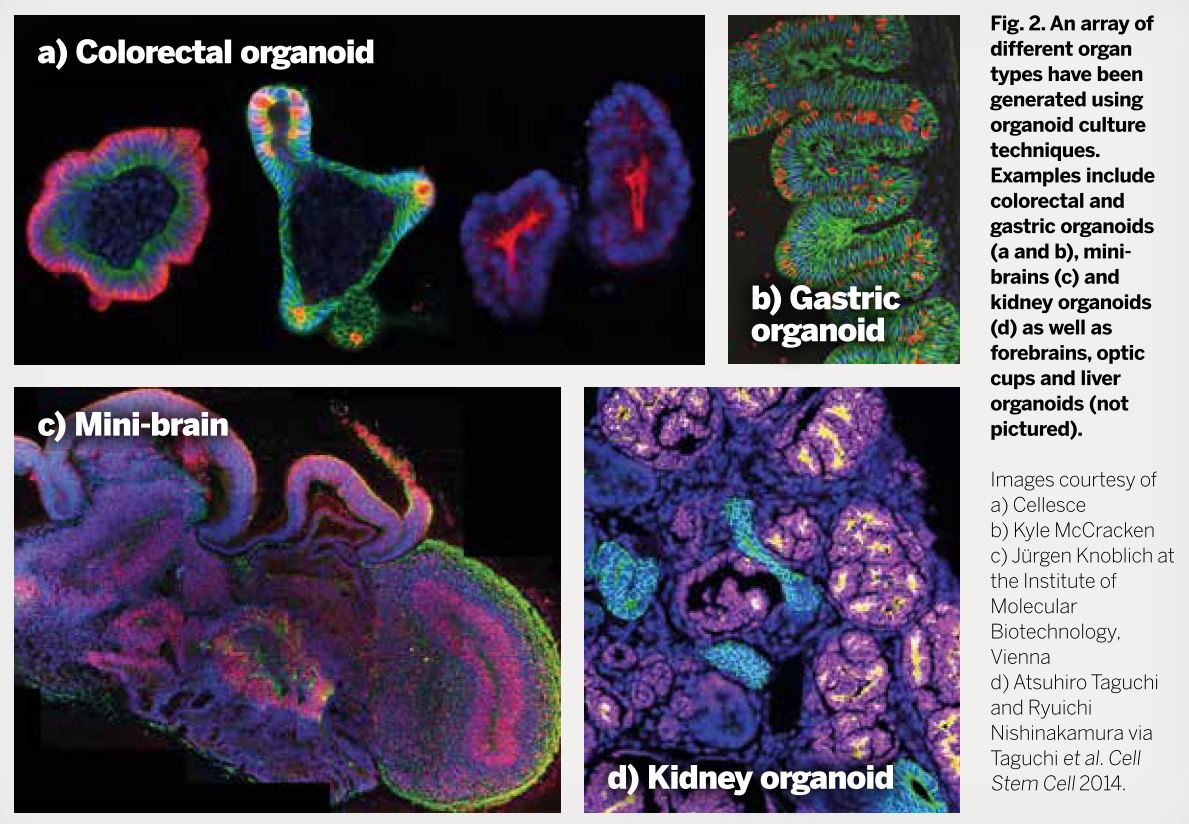 Fig. 2 - (Click to enlarge). An array of different organ types generated using organoid culture techniques.
Fig. 2 - (Click to enlarge). An array of different organ types generated using organoid culture techniques. As well as modelling the progression of disease states, organoids may have uses in regenerative medicine. Using cellular material from patients through generating iPSCs, a patient's own cells can be guided towards the required cellular lineage or fate, and the organoids that are formed from this tissue source can be transplanted back into the patient, such as in the use of liver organoids to treat liver cirrhosis[7]. This has the added benefit of avoiding tissue rejection associated with transplants from other individuals and abrogates the necessity for a lifetime of immunosuppressant drugs to counteract organ rejection.
Organoids have significant advantages over 2D techniques in terms of studying the spatial organisation and development of organs and tissues, significantly so when compared with embryoid body culture. However, with the exception of the Zika virus study mentioned above, there are key issues in their reproducibility: in other words, under the same experimental conditions, there is significant variation between the observed outcomes, and the frequency of these outcomes is low[8].
If organoids are to be used as model systems for disease states or understanding development, as well as being used clinically for drug screening or for regenerative medicine, it is essential that they are reliable, reproducible and quantifiable. Advances need to be made so that organoid systems can be precisely controlled and manipulated with minimal experimental variation.
Gastruloids – embryonic organoids
With this in mind, our laboratory in Cambridge developed a new, highly reproducible and tractable tissue-culture technique using mouse ESCs to study early mammalian development and axial patterning[5,9–11] (Fig. 2). Building on earlier observations with the P19 embryonal carcinoma cell line[4] and the suspension culture techniques used by other groups[12], we aggregated small numbers of mouse ESCs in suspension to form spherical structures on the same scale as the early mouse embryo at the blastocyst stage. This is in contrast to most other organoid systems or embryoid bodies where larger numbers of cells are typically used.
Over time, and under appropriate signalling conditions, these spherical aggregates or, rather, embryonic organoids, begin to show something remarkable, undergoing many of the morphological and patterning events of the early embryo. Furthermore, they undertake a process similar to gastrulation in the embryo, generating cell types that correspond to the three germ layers [9,11,13].
It is the combination of these traits that gave rise to the name of these embryonic organoids: gastruloids.
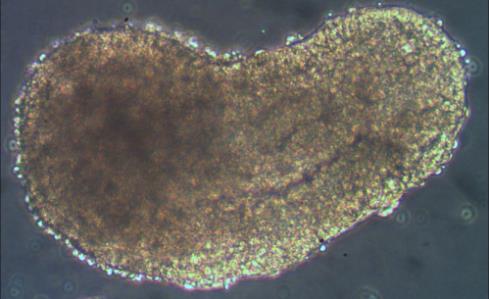
A striking aspect of gastruloids (see opening image) is the finding that they can form a structure similar to one that forms only in mammalian embryos: the node[11]. The node is a key signalling centre in mammalian embryos and is important for identifying which side of the embryo is left or right, and the disruption of the left-right patterning causes severe birth defects, usually leading to chronic heart disease. Presently, the only way to study the effect of left-right axis disruption is through animal models, so the gastruloid system offers an attractive window into studying this process cheaply and without using animals.
Interestingly, the differences between gastruloids and the embryo have already helped provide an alternative interpretation of the function of certain embryonic tissues[11].
Ethical minefields
Despite the above examples, a burning issue still lingers: organoids don't fully equate to human development. Even though many signalling pathways and patterning mechanisms have been conserved throughout evolution, other animals may do things differently to the human embryo, and disease-modelling using material from tissues, organoids and embryos that are different to that of human origin may give rise to errors in our understanding that will impact the effectiveness of treatment.
An approach more indicative of human development will eventually be required to fully appreciate how we develop. However, there are limits on what is permissible regarding experimentation on human embryos and for how long scientists can culture human embryos. Presently, human embryos can only be cultured for a limited period of time, either for up to 14 days or until the 'primitive streak' structure forms, which signifies the start of gastrulation[14].
Many of the questions regarding the development of our organs and how human embryos are patterned occur after this point. There are many ethical questions surrounding the use of ESCs, which must be obtained from human embryos that are then destroyed.
It is here that organoids have the potential to overcome this barrier in an ethically acceptable manner and (in terms of regenerative medicine and tailored treatments) in ways more tailored to specific disease states in patients. Generating organoids from current stocks of human ESCs or from human iPSCs, we will be able to limit the requirement for further human embryos to study early human development (in the case of the former) and also take starting material directly from patients to study their disease in a genetic background identical to the patient's or provide source material for possible regenerative medicine (in the case of the latter).
On a final note, it is important for us to keep in mind potential problems that may arise in generating embryonic structures in culture from human ESCs/iPSCs, as recently pointed out by Aach et al (2017). As organoids have the potential to form later embryonic structures without progressing through a primitive streak, it may arise that we have created a structure that has neurulated, generating something that has the potential to feel pain or develop sentience[14] (hence this is one of the reasons for stopping human embryo work at the 14-day/primitive streak stage). Current guidelines regarding the use of human embryos cannot apply in this case since the primitive streak hasn't formed.
Research and bioethical communities may need to find new ways to address these ethical problems with their findings disseminated and communicated with the wider public. It may be the case that new legislation should be drawn up to reflect both our new scientific understanding based on what our experiments tell us about development, and our changing perspectives on what is ethically acceptable[14].
There are many methods used to study development, generating structures that can be used both as a substitute for the embryo in research and for regenerative medicine and modelling disease progression. However, it is essential that we bridge the gap between what we are able to accomplish through our experiments and the translation of our knowledge to the public, without overselling what we hope to achieve or what is actually possible[8].
Still, as great progress has been achieved in a relatively short space of time, it will be interesting to see what new developments will arise and how this highly evolving field can translate its findings for regenerative medicine and modelling diseases.
Dr David A Turner CBiol MRSB is an NC3Rs David Sainsbury Research Fellow working in Alfonso Martinez Arias' laboratory at the University of Cambridge. He uses the gastruloid model system to study development in the mammalian embryo.
1) Turner, D. A. et al. Wnt/β-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development 141, 4243–4253 (2014).
2) Turner, D. A. et al. An interplay between extracellular signalling and the dynamics of the exit from pluripotency drives cell fate decisions in mouse ES cells. Biology Open 3, 614–626 (2014).
3) ten Berge, D. et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508–518 (2008).
4) Marikawa, Y. et al. Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis 47, 93–106 (2009).
5) Turner, D. A. et al. Organoids and the genetically encoded self-assembly of embryonic stem cells. Bioessays 38, 181–191 (2016).
6) Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
7) Willenbring, H. & Soto-Gutierrez, A. Transplantable liver organoids made from only three ingredients. Stem Cell 13, 139–140 (2013).
8) Huch, M. et al. The hope and the hype of organoid research. Development 144, 938–941 (2017).
9) van den Brink, S. C. et al. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231–4242 (2014).
10) Baillie-Johnson, P. et al. Generation of aggregates of mouse embryonic stem cells that show symmetry breaking, polarization and emergent collective behaviour in vitro. J .Vis. Exp. (2015). doi:10.3791/53252
11) Turner, D. A. et al. Gastruloids develop the three body axes in the absence of extraembryonic tissues and spatially localised signalling. BioRxiv (2017). doi:10.1101/104539
12) Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
13) Turner, D. A. et al. Interactions between nodal and wnt signalling drive robust symmetry-breaking and axial organisation in gastruloids (embryonic organoids). BioRxiv 1–31 (2016). doi:10.1101/051722
14) Aach, J. et al. Addressing the ethical issues raised by synthetic human entities with embryo-like features. Elife 6, e20674 (2017).