The road to replacement
Genevieve Barr and Alice Carstairs explore the latest innovations and support to help scientists replace, reduce or refine the use of animals in research
20th February 2023
The 3Rs – replacement, reduction and refinement – are the principles that underpin the ethical use of animals in scientific research and testing. Replacement addresses animal use before an experiment even begins, asking whether animals are needed to achieve the scientific objectives or whether there is a viable alternative approach.
Advances in technology and engineering are manifesting in a new generation of non-animal technologies that can replace the use of animals in experiments where historically there have been no alternatives. Increasingly complex laboratory-based in vitro techniques are being developed where cells can be grown in 3D alongside multiple other cell types to better recapitulate in vivo biology.
The possibilities and power of in silico techniques continue to increase, with mathematical modelling or computer simulations able to provide new insights into existing datasets, create a digital model that can be interrogated, or predict the properties of proteins or drugs. In fact, non-animal technologies can address some of the limitations of using animals in experiments, with in vitro or in silico replacements potentially providing more physiologically relevant, predictive, robust and replicable results.
Sometimes it is not possible to fully replace the use of animals, but researchers can consider what is called a ‘partial replacement’. Could they use a different in vivo model that, according to current scientific thinking, is not capable of experiencing suffering? For example, experiments using fish and chick embryos in early life stages or invertebrates such as fruit flies (Drosophila), nematode worms or social amoebae. Ex vivo models, in which an animal is culled to provide cells or tissue samples for experiments, are another partial replacement.
The National Centre for the 3Rs (NC3Rs) works with the scientific community to embed the 3Rs into practice, providing funding for research and innovation, guidelines for best practice, and information and advice for researchers and laboratory animal care staff. Around two-thirds of our budget for funding research has been committed to projects dedicated to replacing animal experiments.
In October 2022 we reached an important milestone, having committed over £50m to projects that replace the use of animals across a range of institutions and scientific disciplines. Within the replacement projects in our portfolio, infection and infectious disease is the most studied area (see ‘Replacing animal use in infectious disease research’, below).
Awarding excellence
Each year the NC3Rs awards an International Prize, sponsored by GSK, to an outstanding original research paper with major impacts on the 3Rs. Both this year’s 3Rs prize winner and highly commended runner-up described promising replacement technologies.
The prize was awarded to Dr Daniel Ferreira from the Instituto de Investigação e Inovação em Saúde in Portugal for his method to produce organ-on-a-chip systems. Organ-on-a-chip is a sophisticated in vitro technique consisting of tissues or cells grown on microfluidic plastic chips to create miniature representations of organs in the lab. The latest version of this technology enables multiple organs to be represented on a single chip, creating a body-on-a-chip.
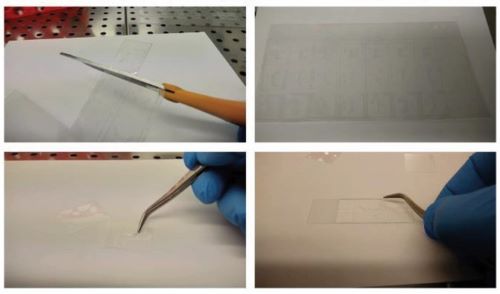 Dr Ferriera's fine organ on a chip systems can be fabricated with commercially available materials and equipment.
Dr Ferriera's fine organ on a chip systems can be fabricated with commercially available materials and equipment.Organ- and body-on-a-chip systems are very good models of human biology and have been successfully used to replace animals in complex disease modelling, personalised medicine, and toxicology and safety studies. Unfortunately, their utility has been limited by the high cost, long time frame and specialised equipment required to produce these complex and precise microphysiological systems.
However, in his award-winning paper Ferreira describes a method for organ-on-a-chip systems to be designed, developed and printed with commercially available equipment and materials.
By overcoming the resource barriers associated with their production, Ferreira’s work will enable more researchers to access this technology and replace the use of animals in their research.
Dr Ben Newland from Cardiff University was highly commended for his ex vivo model of multiple sclerosis (MS). As MS develops, the immune system degrades the myelin protein that surrounds neurons, forming gaps in the brain called focal lesions.
This process can be studied in the laboratory by treating brain tissue slices, typically from rodents, with chemicals to break down the myelin protein.
Newland’s work improves on the existing method, which involves treating an entire tissue slice with these chemicals, by developing a porous gel that soaks up the demyelinating chemicals and can be applied to specific areas of brain tissue. This recreates the focal lesions seen in MS patients, meaning results generated using Newland’s method are more relevant to human disease.
Newland’s improved ex vivo model will help to further replace animal experiments. Newland has been awarded NC3Rs funding to teach his method to collaborators at Queen’s University Belfast, and is creating detailed protocols and training videos to share this technique more widely.
Innovation versus inertia
Implementing non-animal technologies means introducing change, and adapting to new ways of doing things can be difficult for both organisations and individuals. In science, certain experimental protocols have been in use for years or even decades, and a researcher may have built their entire career publishing results from a particular animal model. Breaking away from established approaches that are widely accepted by the institutions that fund their research, and peers and journals that review and publish their work, may be viewed as a significant risk.
To effect the wholesale change required to accelerate the adoption of replacement technologies, we work with funding bodies, ethics boards, learned societies and journals, helping to raise awareness of the suitability and scientific value of non-animal approaches. We want to ensure researchers have all the information they need and the confidence to choose the best model to address their scientific question.
Improving awareness and confidence in new approaches is one way we can tackle the ‘3Rs valley of death’: the often long lag between the development of new technologies and their adoption into routine use.
Another is through our links with industry, including the pharmaceutical, biotechnology and agricultural sectors, which are critical to accelerate the use of non-animal technologies. Our CRACK IT open innovation programme funds collaborations between industry, academics and small and medium-sized enterprises to solve business and scientific challenges.
The most recent challenges, launched in 2021 and 2022, aim to develop novel replacement technologies. For example, the Virtual Second Species Challenge seeks to use advanced computational and mathematical modelling to replace some of the in vivo toxicity studies required for drug development. Typically, both rats and dogs are used for pre-clinical studies to investigate the toxic effects of new drugs and identify a safe starting dose for human clinical trials. The goal of this particular challenge is to develop a suite of virtual dog tissues and organs that can predict the toxic effects of new drugs, building confidence in in silico models as replacement technologies and developing the evidence to support the use of a single rodent species for chronic toxicity studies.
Launched in July 2022, the aim of the T-ALERT Challenge is to develop an in vitro assay to assess the safety of cancer immunotherapies. T cell therapies are a promising new approach that targets the tumour using modified immune cells that specifically attack cancer cells, but there is a risk that the process of modifying the T cells could cause them to become tumourigenic (cancer-causing). This challenge seeks to evaluate this risk using an in vitro assay that is cheaper, faster and more reliable than current in vivo experiments that require 30 to 40 animals, last for two to six months, and may have limited value due to differences in human and animal immune systems.
Into the future
Since the NC3Rs was launched in 2004 we have come a long way on our mission to embed the 3Rs in the use of animals in scientific research and testing. Replacement takes centre stage in the NC3Rs’ vision for the future of animal research, and in 2022 we published an updated NC3Rs strategy, which sets out five main initiatives to achieve our goal of accelerating the use of models, tools and technologies to replace animal studies over the next three years.
These include committing to providing at least £6m annually across our funding schemes for replacement projects. Our PhD studentship scheme will only fund projects with a replacement impact and will offer targeted training to support the future skills base in replacement technologies. Early career scientists are often more responsive to trying new approaches and we aim to instil the principle of replacement into the next generation of scientific leaders.
We are also accelerating the uptake of replacement alternatives by focusing on areas that have been resistant to change. For example, antibodies are a critical detection tool used in many in vitro experiments to visualise and quantify proteins, but they are typically still produced from animal blood products or bone marrow, despite the high specificity and reproducibility of antibodies derived from non-animal sources. We are working with the scientific community to tackle the barriers to adoption of non-animal-derived antibodies, including commercial (supply and demand), scientific, practical (lack of experience and cost) and cultural (reluctance to change).
We will also be developing a new set of recommendations to improve the reliability and reproducibility of in vitro experiments, which offer significant replacement opportunities. The RIVER (Reporting In Vitro Experiments Responsibly) recommendations aim to raise reporting standards and build credibility and confidence in non-animal technologies.
Finally we are developing guidance to help researchers to find and use non-animal technologies. There is increasing interest from the scientific community in using replacement technologies, but the guidance and databases researchers may use to search for these technologies are often out of date and difficult to access, and their content may be limited to specific technologies or disciplines.
We have reached a bridge on the road to replacing animals in research. On one side are innovative non-animal technologies, increasingly able to not only replace the use of animals but provide results more relevant to human health and disease, faster and more cheaply. We are building the bridge to connect these replacement technologies to scientists, ethical review boards, journals and regulatory bodies that perform, approve or require experiments using animals.
Professor Aaron Maule at Queen’s University Belfast has established a method of growing and genetically modifying liver fluke in the lab, replacing the need to infect rodents in order to produce these parasitic worms. This technique has replaced the use of over 200 rodents a year in his laboratory.
Mice are commonly used to study tuberculosis (TB) but they do not develop granulomas – clumps of immune cells that aggregate around the TB bacteria – which are a key characteristic of human infection. Professor Paul Langford of Imperial College London showed that wax moths develop granuloma-like structures when infected with TB, and can be used instead to study TB infection and drug resistance. This model replaces up to 200 mice per drug screened and screening takes days rather than weeks.
Dr Gyorgy Fejer from the University of Plymouth studies how lung macrophages protect us from the bacteria and viruses we breathe in. Typically, these cells must be isolated from mouse organs or bone marrow, but Fejer has developed a technique to continuously grow lung macrophages in the lab. His method produces 30 million lung macrophages from one flask, compared with the 0.3 million cells that can be isolated from one mouse, replacing the use of 100 mice a year in his laboratory.
Find out more about the NC3Rs work by visiting www.nc3rs.org.uk or follow them on Twitter and LinkedIn.
Genevieve Barr is science manager for communications at the N3CRs.
Alice Carstairs is science manager for research funding at the N3CRs.


