Crabs, cures and conservation

8 September 2025
Every year the bright blue blood of thousands of horseshoe crabs is harvested for use in pharmaceutical testing. Richard Gorman, Jolie Crunelle and Kristoffer Whitney explore why the practice continues despite synthetic alternatives being available
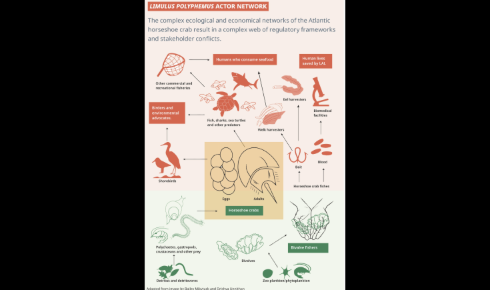
Fossil records suggest horseshoe crabs have survived largely unchanged for over 400 million years – hence why they are commonly referred to as ‘living fossils’. Not technically crabs at all (they have their own order, Xiphosura), these ancient organisms play a key role within ecosystems, providing food for shorebirds, finfish and sea turtles, and are themselves a habitat for ‘hitchhikers’ such as sponges, worms, crustaceans, molluscs and snails, which attach themselves to the ‘crabs’ over their relatively long lives (up to 18 years).
Interest in horseshoe crabs began to grow towards the end of the 19th century – the organisms were large, readily available and easy to maintain in laboratory aquaria. The establishment of the Marine Biological Laboratory in 1888 at Woods Hole, Massachusetts, close to the breeding grounds of the Atlantic horseshoe crab (Limulus polyphemus), inspired a variety of early studies on the species.
The animals’ sheer size – females can grow to up to two feet long – made them well suited to morphological and physiological research, and studies of the horseshoe crab eye contributed to the 1967 Nobel Prize in Medicine for discoveries on the physiological and chemical processes of vision.
However, it was the crabs’ unusual blood – which turns blue in the presence of oxygen due to copper-rich proteins – that propelled them to the pharmaceutical centre stage.
 Although horseshoe crabs are released after being bled for LAL extraction, many die, and the effect on those that live is disputed. The Atlantic horseshoe crab is classed as ‘vulnerable’ to extinction by the IUCN.
Although horseshoe crabs are released after being bled for LAL extraction, many die, and the effect on those that live is disputed. The Atlantic horseshoe crab is classed as ‘vulnerable’ to extinction by the IUCN.
In the 1950s, American biologist Frederik Bang had speculated that species of ancient origin might reveal primitive immunological functions. Collaborations with haematologist Jack Levin revealed the key factor that caused horseshoe crabs’ blood to clot after injury was bacterial endotoxin, composed of lipopolysaccharides found on the outer membrane of Gram-negative bacteria.
By 1968, Levin and Bang had recognised that the sensitivity of this system could be used as the basis of a fast, reliable and convenient in vitro test for the presence of bacterial endotoxin. Although ubiquitous in the natural environment, endotoxins are capable of causing fever and other dangerous immune responses when released into the mammalian bloodstream. Not destroyed by conventional sterilising or heat-treating protocols, endotoxins represent a huge challenge for both human and veterinary medicine, and agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency require that any product that comes into contact with the bloodstream or spinal fluid must be below a certain threshold of endotoxin. Levin was able to show that horseshoe crabs’ blood-clotting mechanism was contained within highly mobile immune cells known as amoebocytes. Lysis of the cells created a substance that was extremely sensitive to the presence of endotoxin in vitro, and the Limulus Amebocyte Lysate (LAL) test was born. It revolutionised medicine.
The previous method for quantifying the presence of dangerous endotoxin in pharmaceutical products had been the in vivo Rabbit Pyrogen Test (RPT). The move to LAL for endotoxin testing enabled the phasing out of large colonies of rabbits (and of the technicians responsible for injecting the rabbits with samples).
Welfare concerns
While the move to LAL is often held up as a unique example of a successful in vitro replacement of an animal toxicity test, the LAL test still requires animals – albeit ones that have become hidden in the supply chain to the point where many LAL users today aren’t necessarily aware of the source of the reagents in their laboratories.
To meet the demand for endotoxin testing today, over 700,000 crabs are removed annually from the east coast of the US to be ‘bled’ for the production of LAL. Bleeding the horseshoe crab involves bending them at their ‘hinge’ – the large lateral join between their prosoma (head) and abdomen – and placing them in a rack. A sterile needle is then inserted through the membrane in the hinge, allowing blood to flow into the container until the flow stops naturally.

Bleeding a horseshoe crab means bending them at their ‘hinge’, placing them in a rack, inserting a needle, then allowing blood to flow into a container
Although the exact amount of blood extracted from the crabs is variable (and controversial, given the LAL industry’s tendency toward secrecy), initial industry estimates and academic studies have suggested that on average about 30% of an animal’s blood is removed during bleeding¹,². In public, however, LAL industry representatives have more recently suggested that only 15% of an average horseshoe crab’s blood is collected. Openness and transparency on the use of animals has not permeated into discussions about horseshoe crab use as it has in other areas of the biomedical sector. Other than provisions in the industry’s best management practices on how the animals are collected and handled, horseshoe crabs’ ‘welfare’ is rarely discussed.
Many of the horseshoe crabs die in the process, despite the practice being framed as a catch-and-release fishery. The Atlantic States Marine Fishery Commission, which regulates the sector, assumes a 15% mortality rate for bled and released crabs³. However, this model is highly debated and contested within the published literature⁴,⁵,⁶.
Along with mortality rates, there is growing concern about the ‘sub-lethal’ effects of biomedical bleeding – the impact of stress from capture, transport and bleeding, particularly on reproductive fitness. The continued use – and potential rise in use given increasing global demand for pharmaceuticals – of horseshoe crabs has prompted growing questions around welfare and sustainability.
Synthetic solution
In the 1990s, Jeak Ling Ding and Bow Ho at the University of Singapore developed a recombinant alternative to LAL from the Mangrove horseshoe crab (Carcinoscorpius rotundicauda). Known as recombinant Factor C (rFC), initial uptake of this recombinant alternative was limited by the market dominance of the LAL test and a lack of regulatory flexibility to consider alternatives.
Since then, several LAL producers have developed their own versions of rFC, as well as recombinant alternatives that mimic the entire LAL cascade, not just the Factor C reaction – often referred to as recombinant cascade reagents (rCR). The development of the Monocyte Activation Test (MAT), which uses human whole blood or human monocytes to detect pyrogenic (fever-causing) substances, has provided another potential alternative to LAL. Many advocates suggest that the ideal, animal-free pyrogen testing regime would be a combination of rCRs for endotoxins and MAT for non-endotoxin pyrogens.
So if there are multiple alternatives available, then why are we still bleeding horseshoe crabs?
Red tape and blue blood
European regulators have allowed the use of synthetic alternatives to the horseshoe crab test since 2016, as long as companies carry out extra checks to show they perform reliably. And in 2021 rFC was officially added to the list of approved methods for testing medicines. Nevertheless, many official documents for individual drugs still specifically mention the LAL test rather than the other tests that are available.
In 2024 revised versions of two widely used European Pharmacopoeia monographs allowed for the use of rFC for testing pharmaceutical waters without a side-by-side comparison. While the United States Pharmacopeia has issued similar revisions to its guidance this year that give rFC equal status to LAL, the FDA has chosen not to license rFC or rCRs the way it does LAL, which means there are still extra steps to validate the use of recombinant products.
In short, animal-free bacterial endotoxin testing remains an ‘alternative’ method worldwide. However, our research has shown that the slow pace of regulatory acceptance is not the sole barrier to the industry moving away from horseshoe crab-derived reagents⁷,⁸,⁹.
Catch, conserve or kill?
The Atlantic horseshoe crab is classed as ‘vulnerable’ by the International Union for Conservation of Nature, which means the species is threatened with extinction unless the circumstances that are threatening its survival and reproduction improve. Horseshoe crabs are caught in huge numbers for use as bait for fishing eel and whelk, and many in the biomedical sector feel aggrieved about the attention that their relatively small use of crabs attracts in contrast to the bait industry, which uses far more crabs and kills 100% of them.
 Huge numbers of horseshoe crabs are also caught for use as fishing bait
Huge numbers of horseshoe crabs are also caught for use as fishing bait
There are even concerns that turning to synthetic alternatives could, ironically, actually result in more harm to horseshoe crab populations if they are no longer seen as a high-value part of the biotech economy and thus worthy of stewardship.
The idea that a species is only protected if it can be exploited doesn’t sit well with most conservationists. But our research has found that many in the pharmaceutical sector are reassured by biomedical companies’ messaging around investment in horseshoe crab conservation, such as funding for habitat restoration, hatcheries and egg-rearing programmes⁷,⁸,⁹. When using LAL is positioned as helping fund the protection of horseshoe crabs, there is less incentive to switch to a recombinant alternative.
To complicate things further, the Asian species Tachypleus tridentatus (tri-spine horseshoe crab) is used to produce Tachypleus Amebocyte Lysate (TAL), used by Asian and Pacific-based pharmaceutical manufacturers. Unlike LAL harvesting, this is not a catch-and-release fishery, and Tachypleus are considered ‘endangered’ due to overharvesting and high mortality. Conservationists in North America worry that as declining populations of Asian horseshoe crabs limit TAL production, LAL manufacture may be increased to compensate, further stressing vulnerable Limulus populations.
The extent to which the biomedical use of crabs has an impact on their populations remains contested, with different sectors and stakeholders attempting to apportion blame for any vulnerability or decline on different factors.
There are parallels – and perhaps optimism too – to be found in history. The replacement of the RPT by LAL as the gold standard for endotoxin detection also involved a lengthy and complex process, and industry acceptance and adoption was slow while data was gathered. It should be noted, however, that the economic incentive to switch from LAL to recombinants is not as pressing as the switch from RPT to LAL 50 years ago, when rabbit colonies were expensive to maintain. If industry is to invest the effort required to switch from LAL to rCRs, there will need to be incentives above and beyond the financial.
A public issue
We are all, as users of medicines, consumers of horseshoe crab blood. Yet there is little research about public attitudes to horseshoe crabs and their use, and little opportunity for the public to shape debates. However, we have observed a growing public awareness of and interest in these animals. Stories about horseshoe crabs in the press and media generate huge interest, attention and discussion. The topic is certainly of public interest, and growing.
 Despite synthetic alternatives, horseshoe crab blood is still widely used by the pharmaceutical industry
Despite synthetic alternatives, horseshoe crab blood is still widely used by the pharmaceutical industry
Replacing the crab, even once regulation takes shape more firmly and openly, will remain challenging, and requires cultural change beyond merely technical ability or economic incentives. Interdisciplinarity and collaboration can help make this largely invisible issue more visible, and concerned citizens can lobby for additional regulation and put pressure on the pharmaceutical sector to pursue culture change.
Public interest could, in fact, be the deciding factor that breaks pharmaceutical companies out of their inertia to adopt these new technologies to create more ethical and sustainable futures for humans.
1) Charles River Laboratories. Horseshoe Crab Conservation.
2) Moore, L.J. Catch and Release. NYU Press (2017).
3) Atlantic States Marine Fisheries Commission. Best Management Practices for Handling Horseshoe Crabs for Biomedical Purposes (2023).
4) Rudloe, A. The effect of heavy bleeding on mortality of the horseshoe crab, Limulus polyphemus, in the natural environment. J. Invertebr. Pathol. 42(2) 167–176 (1983).
5) Leschen, A.S. & Correia, S.J. Mortality in female horseshoe crabs (Limulus polyphemus) from biomedical bleeding and handling: implications for fisheries management. Mar. Freshw. Behav. Physiol. 43(2), 135–147 (2010).
6) Anderson, R.L. et al. Sub-lethal behavioral and physiological effects of the biomedical bleeding process on the American horseshoe crab, Limulus polyphemus. Biol. Bull. 225(3), 137–151 (2013).
7) Crunelle, J. & Whitney, K. Regulating horseshoe crabs: sustainability and public health. OSF Preprint (2023).
8) Gorman, R. Atlantic horseshoe crabs and endotoxin testing: perspectives on alternatives, sustainable methods and the 3Rs (replacement, reduction and refinement). Front.Mar. Sci. 7, 582132. (2020).
9) Gorman, R. Outside of regulations, outside of imaginations: why is it challenging to care about horseshoe crabs? Researching Animal Research, 57–79 (Manchester University Press, 2024).
Dr Rich Gorman is assistant professor in ethics and social science at Brighton and Sussex Medical School.
Jolie Crunelle recently graduated the Rochester Institute of Technology with a BSc in biomedical engineering and an MS in science, technology and public policy.
Dr Kristoffer Whitney is associate professor in the Department of Science, Technology and Society at Rochester Institute of Technology, New York.