Buried Alive

Above: A rainbow captured over the drilling rig of the research vessel JOIDES Resolution. Image courtesy of IODP JRSO
Yuki Morono explains why the discovery of living bacteria in 100 million year old sediment may force us to rethink the minimum levels of energy and nutrients required to power life
Feb 22nd 2021
Casual, intermittent fasting has become a popular health trend in recent times, but imagine being stuck in your wardrobe without enough food for millions of years. A recent study by myself and colleagues at earth science and oceanography institutes in Japan and the US[1] showed that microbes deep beneath the ocean floor, trapped without ready access to nutrients for more than 100 million years, could be revived and multiplied in the laboratory.
The deep subseafloor appears barren and desolate, but may hold between 10% and 30% of all microbial biomass on Earth. Marine snow – a steady shower of organic detritus falling from the photosynthetic sea surface – is the main source of nutrients for these microbes living in the sediment at the bottom of the ocean. However, the subseafloor of the South Pacific Gyre (SPG) is different. The SPG is far from any continents or productive areas of ocean, kept isolated from the rest of the world by continuous circular currents and winds, and is regarded as Earth’s largest ocean desert. It has the lowest productivity of any ocean region on Earth and nutrients on the subfloor are extremely limited.
 Microbiologists Yasuhiko Yamaguchi, Yuki Morono, Laurent Toffin and Steven D’Hondt examine the cores. Image courtesy of IODP JRSO.
Microbiologists Yasuhiko Yamaguchi, Yuki Morono, Laurent Toffin and Steven D’Hondt examine the cores. Image courtesy of IODP JRSO.Aiming to explore the question of whether life could exist in such a nutrient-limited environment, the drilling expedition of the Integrated Ocean Drilling Program (IODP) was initiated in 2010. Aboard research drillship JOIDES Resolution we obtained numerous sediment cores from 100 metres below the seafloor of the SPG, nearly 6,000 metres below the ocean’s surface.
The accumulation of marine snow and particles carried by the wind and ocean currents over huge periods of time are the main constituents of subseafloor sediment. The deeper you drill, the older the sediment is. This slow but constant accumulation of sediment makes it possible to obtain subseafloor sediment formed as long as 100 million years ago.
The sediments we obtained were composed of very fine (~1 micrometre) clay particles. The calculated space between particles was also very fine – narrower than the size of the microbes we discovered. Therefore these microbes, once settled in the sediment, should in theory have been trapped and unable to move from their original position since burial. We also found that oxygen was present in all of the cores, suggesting that if sediment accumulates slowly on the seafloor, at a rate of no more than a metre or two every million years, oxygen can continue to penetrate from the seafloor down to the thick layer of igneous rock below the sediments. Such conditions appear to make it possible for aerobic microorganisms to survive for geological timescales of millions of years.
There were two critical things we were careful about in our experiments. First, we had to avoid contamination with microbes from the surface world in our samples. We carefully checked for sampling-derived contamination (i.e. during drilling) by using a sensitive chemical tracer that detects any intrusion of the fluid used for drilling. In the lab all the sample handling was done using sterilised tools. Later, DNA-based microbial taxonomy analyses helped verify the microbial samples were not known strains of bacteria from the surface.
The other key element was the sample-handling temperature. At the seafloor the water temperature is around 1°C, with the temperature rising with increasing sediment depth up to around 6°C at the deepest part. After many millions of years at this temperature our ambient temperatures would not be a comfortable environment for any microbes found in the samples. For this reason I spent many hours in the cold room at the bottom of the ship preparing the incubation experiment for resuscitating the microbes in the sediment. After being in this cold and contained space for such a long time – for up to 10 hours a day – I started to feel like I understood how it felt to live in the subseafloor environment.
 Core samples half-cut for onboard subsampling. Image courtesy of IODP JRSO.
Core samples half-cut for onboard subsampling. Image courtesy of IODP JRSO.Based on the nutrient-limited nature of sediments, we assumed any microbes present would be in a state of near-dormancy and not easily revived. However, the microbes started to incorporate nutrients within 21 days of the start of incubation, including in the sediment samples deposited 100 million years ago. These samples were found within tens of centimetres above the basement rock, which was aged at 103 million years.
Within 68 days the cells had increased by up to 10,000 times their original concentration, with an average doubling time of approximately five days. This was an average of 34 times faster than the subseafloor microbes found in anoxic sediments off Japan, which had previously been investigated in the same way. Here, there is an abundance of organic matter in the subseafloor, so it was thought that the microbes might be found with higher activities than in the SPG subseafloor. We were initially taken aback by the results, even skeptical.
But, after careful examination of each step of the experiments, we found that up to 99.1% of the microbes in sediment deposited 101.5 million years ago were still alive – and they were ready for a well-deserved meal.
How is this quasi-suspended animation possible? In 2000 a similar debate was sparked by the apparent revival of bacteria from 250-million-year-old salt crystals[2]. In this case scientists were even more skeptical as it should be theoretically impossible for a microbial cell to stay alive in complete isolation inside a salt crystal.
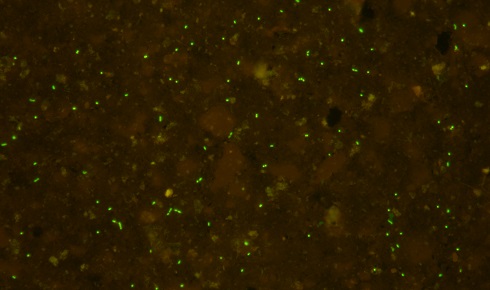 Membrane-trapped fluorescence microscopy image of a sample after cell separation (before cell sorting).
Membrane-trapped fluorescence microscopy image of a sample after cell separation (before cell sorting).All living organisms require a certain amount of energy to maintain their body and its constituents. For example, DNA and protein are relatively unstable molecules in cells and will degrade with time if not repaired – organisms cannot simply maintain their living form in complete isolation. In SPG sediments, although the microbes are trapped for a huge period of geological time, the sediment still has narrow channels that allow diffusion of water-soluble molecules such as oxygen, so the sediment is not shut off from the world. Also, there is a very low but detectable level of organic matter, a source of energy for aerobic respiration. Collectively, the microbes revived from SPG sediment had been starved, but not completely.
The availability of energy, however, is extremely scarce: previous calculations[3] estimate that the power available in the SPG subseafloor environment is at least two orders of magnitude lower than the lowest power known to sustain life in the literature. The results call into question the power limit of life and more recent work[4] suggested that, globally, subseafloor microbes must subsist under lower levels of energy availability than previously thought.
The nutrition environment of the microbes in SPG sediment is far below the level that would allow individuals to ‘grow’ or replicate. However, it is not certain that the individual cells in our sample were 100 million years old: it may have been possible for them to divide when they are at a few tens of centimetres below the seafloor. Then, extreme nutrient limitation further down in the sediment would restrict their metabolism and bring them to a state that could just about maintain their structure. In this case it enabled them to survive until a drill bored through the Earth and took them to our lab and a world of abundant nutrients.
 Yuki working on subsampling of the core samples in the catwalk of the Joides Resolution.
Yuki working on subsampling of the core samples in the catwalk of the Joides Resolution.In 1955 Earth’s known biosphere went down only seven metres from the seafloor[5]. From the late 1980s a flurry of discoveries expanded our recognition of deep subseafloor biosphere down to 2.5km from the seafloor[6], and more recently to a sediment age of 100 million years.
For deep biosphere research 2020 was a big year. Alongside our work, Japanese research also isolated the primordial life form Asgard archaea from deep sea sediments[7], an organism at the prokaryote–eukaryote interface that could help to explain the rise of complex life. Further research has been published on the agglomeration of microbes in the crack of deep sub-seafloor rocks[8]. Two important new papers suggest life in the subseafloor is far more diverse[9] and can be found at far hotter deep locations[10] than previously thought.
Now researchers are discussing a framework for scientific ocean drilling towards 2050. One of the strategic objectives is ‘habitability and life on Earth’, and the long-term transdisciplinary research target is ‘exploring life and its origins’. The story of life in the subseafloor environment is to be continued.
A behind-the-scenes video on this JAMSTEC research can be found at bit.ly/deepseamicrobes
Yuki Morono is a microbiologist with the Japan Agency for Marine-Earth Science and Technology (JAMSTEC).
1) Morono, Y. et al. Aerobic microbial life persists in oxic marine sediment as old as 101.5 million years. Nat. Commun. 11, 3626 (2020).
2) Vreeland, R. H. et al. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407(6806), 897–900 (2000).
3) LaRowe, D. E. & Amend, J. P. Power limits for microbial life. Front. Microbiol. 6, 718 (2015).
4) Bradley, J. A. et al. Widespread energy limitation to life in global subseafloor sediments. Sci. Adv. 6(32) (2020).
5) Morita, R. Y. & ZoBell, C. E. Occurrence of bacteria in pelagic sediments collected during the Mid-Pacific Expedition. Deep Sea Res. 3(1), 66–73 (1955).
6) Inagaki, F. et al. Exploring deep microbial life in coal-bearing sediment down to ~2.5km below the ocean floor. Science 349(6246), 420–424, (2015).
7) Imachi, H. et al. Isolation of an archaeon at the prokaryote–eukaryote interface. Nature 577, 519–525 (2020).
8) Suzuki, Y. et al. Deep microbial proliferation at the basalt interface in 33.5–104-million-year-old oceanic crust. Commun. Biol. 3, 136 (2020).
9) Hoshino, T. et al. Global diversity of microbial communities in marine sediment. PNAS 117(44), 27587–27597(2020).
10). Heuer, V. B. Temperature limits to deep subseafloor life in the Nankai Trough subduction zone. Science 370 (6521), 1230-1234 (2020).


