Breaking down barriers
A better understanding of how bacterial biofilms form is vital to prepare us for future pandemics and the worsening of the antimicrobial crisis, writes Susana Direito
May 31st 2021
In the Spanish flu pandemic of 1918-1919 the majority of the estimated 50 million deaths attributed to the virus were likely to have actually been caused by so-called secondary infections, where bacteria commonly found in the upper respiratory tract proliferate and exacerbate the illness[1]. Inflammation or excessive mucus in the lungs allow ordinarily harmless bacteria in the respiratory tract to form stubborn biofilms and become dangerous and often deadly pathogens, such as Streptococcus pneumoniae, which causes pneumonia.
It is no different with the viral pandemic that has been sweeping around the world since last year. As of April 2021 almost three million people have died from COVID-19 caused by the SARS-CoV-2 virus, and co-infections and secondary infections are a common complication of severe cases. Although the prevalence of these infections is not well understood and seems to vary in different studies, it has been found to be as high as 50% among patients who have died[2,3].
What is a biofilm?
Biofilms were first seen by the inventor of the microscope, Antonie van Leeuwenhoek, in the 1680s, when he observed aggregations of tiny ‘animalcules’ in the thick plaque scraped off his teeth – what we might today call a macroscopic, multispecies biofilm. The films are in fact dynamic three-dimensional structures that can be assembled by a wide range of microorganisms, including bacteria, fungi and protists. A thick and often slimy extracellular matrix fills the spaces between cells, sometimes of multiple species, providing connectivity, stability, and a degree of protection against environmental, chemical and mechanical stresses.
Biofilms can form on almost any surface that microorganisms can colonise: in our own bodies, such as in the lungs, in dental plaque or on medical implants; in streams and rivers; even on our toilets and sinks. Some microbiologists even think that the biofilm might be the default bacterial lifestyle, with free-floating bacterial cells increasingly seen as in a transitional or dispersal stage[4].
The matrix, often formed from a mix of DNA, protein and polysaccharides, can physically and chemically prevent antibiotics from reaching the bacterial cells. This barrier can also prevent the hosts’ immune systems from clearing the infection too, making infections persistent or chronic. What’s more, the close contact between the microbial cells in a biofilm allows them to exchange genetic material and resistance genes, increasing the rate at which antimicrobial resistance arises and spreads.
Wet and nutrient-rich environments are optimal media for biofilm development, meaning most of the human respiratory tract (including the nose, mouth, throat, larynx, trachea, bronchi and lungs) are ideal places for these assemblages to form. Biofilms are thought to form in up to 80% of human infections[5] and play a major role in cystic fibrosis (CF), an inherited condition caused by a mutation in a gene that helps regulate liquid volume on epithelial surfaces[6]. This leads to thick sticky mucus building up in patients’ lungs and digestive system, triggering bacteria (often Pseudomonas aeruginosa) to form biofilms in the lungs[7]. As a result, CF patients face regular and very-hard-to-treat infections.
Biofilms are not only extremely important in medicine, but are also economically important, causing widespread issues in cleaning, food and wastewater processing industries. Given the huge role biofilms play in both bacterial and viral infections and industry, surprisingly little is known about exactly what triggers their formation and how to prevent them forming in the first place.
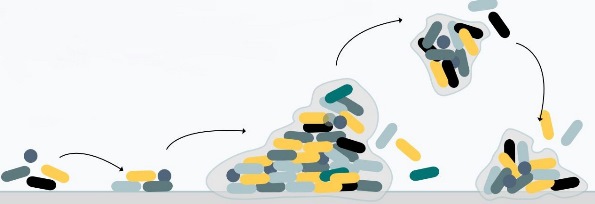 Most microorganisms can attach to and grow upon surfaces (1). After attachment (2), the production of extracellular polymers helps solidify the attachment and link cells in the growing biofilm (3). Cells show marked changes in growth rate and gene transcription compared to free-floating cells. Both single cells and communities of cells may break off to disperse to other locations (4).
Most microorganisms can attach to and grow upon surfaces (1). After attachment (2), the production of extracellular polymers helps solidify the attachment and link cells in the growing biofilm (3). Cells show marked changes in growth rate and gene transcription compared to free-floating cells. Both single cells and communities of cells may break off to disperse to other locations (4).
A biofilm pandemic
Despite the vast amount of research being conducted on understanding, tracking and treating SARS-CoV-2, information on the secondary infections and biofilm formation associated with COVID-19 is limited, with studies often based on small sample sizes. A study in Wuhan in China, where COVID-19 was first detected, revealed that half of patients who died suffered from a secondary infection[3], and we know COVID-19 patients with co-/secondary infections are more likely to die than patients without these infections[8]. Other studies have pointed to co-infections of both bacterial and fungal origin in severely ill patients[9,10].
Pathogens that have been detected in COVID-19 patients include a wide range of bacteria that are known to form biofilms (Acinetobacter baumannii, Klebsiella pneumonia, Staphylococcus aureus (including MRSA), P. aeruginosa, Haemophilus influenzae, S. pneumoniae, Mycoplasma pneumoniae, Chlamydia pneumonia, Legionella pneumophila, Serratia marscecens, Enterococcus faecium, Enterobacter and Chlamydia species), as well as fungi (including Candida and Aspergillus species); and other viruses (other coronaviruses, influenza A and B, rhinovirus/enterovirus, parainfluenza, metapneumovirus, respiratory syncytial and HIV). Polymicrobial secondary infections – i.e. a secondary infection caused by more than one organism – are also possible.
For these reasons treating the viral disease alone cannot be the only focus of our efforts: a better understanding of how biofilms form, how to prevent them and how to break them down could help minimise the number of deaths caused by COVID-19, as well as the impact of the next viral pandemic.
It is not as simple as the administration of antibiotics to patients suffering with COVID infections. As well as the fact that biofilms help prevent the perforation of antibiotics into the colony, the indiscriminate and ineffective use of antibiotics on a pandemic scale will only aggravate the already very serious problem of antibiotic resistance – thus potentially making us even more vulnerable to secondary infections during viral outbreaks. Potential alternatives to antibiotics, including the use of bacteriophages (viruses that infect and kill bacteria) and bacteriophage endolysins (bacteriophage enzymes that digest the bacterial cell walls) can also be hindered by the protective effects of the biofilm, and resistance to these treatments can also arise and spread quickly within biofilms[11].
Combination therapies
Making use of different phage cocktails or combination therapies with antibiotics may be more promising strategies, as well as treatments engineered specifically to penetrate biofilms. Even more important would be the ability to intervene before biofilm formation takes place, or prevent bacteria being able to form biofilms, and finding active anti-biofilm substances is a major goal of current research in microbiology[12]. Different therapeutic strategies are being investigated for different stages of biofilm formation, from prevention of bacterial adhesion to surfaces to substances that can penetrate mature and solidified microbial microenvironments.
A key goal is substances that disrupt the formation of the extracellular polymeric substance (EPS), which forms the tough viscous scaffold of the biofilm. This can only occur when we have a good understanding of how biofilm formation occurs and in what circumstances – a challenging area of study. These are highly heterogeneous and complex communities of living organisms that evolve rapidly. Such research requires contributions from several disciplines, including microbial genetics, microbial ecology, biophysics, soft matter physics, engineering, mathematical modelling and others, and partnerships between academia and industry.
Given the ongoing pandemic, biofilm research has never been as relevant. And a better understanding of biofilms will mean we are better prepared to deal with the inevitable next pandemic and the worsening of the antimicrobial resistance crisis.
The National Biofilms Innovation Centre (NBIC) is currently running a #BiofilmAware campaign to raise awareness of biofilm research, which will include the introduction of the first #BiofilmWeek on 16 August 2021. See www.biofilms.ac.uk/biofilmaware for more information.
Susana Direito is an NBIC research Fellow and part of the Edinburgh Complex Fluids Partnership core team at the University of Edinburgh. Direito has won awards to partner with industry in order to address problems caused by biofilms and to help in the development of disinfection technologies.
1) Morens, D. M. et al. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198(7), 962–970 (2008).
2) Lai, C. C. et al. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 53(4), 505–512 (2020).
3) Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229), 1054–1062 (2020).
4) Boudarel, H. et al. Towards standardized mechanical characterization of microbial biofilms: analysis and critical review. NPJ Biofilms Microbiomes 4, 17 (2018).
5) National Institutes of Health.
6) Davies, J. C. et al. Cystic fibrosis. BMJ (Clinical research ed.) 335(7632), 1255–1259 (2007).
7) Moradali, M. F. et al. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 7(39) (2017).
8) Lansbury, L. et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 81(2), 266–275 (2020).
9) Lai, C. C. et al. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 53(4), 505–512 (2020).
10) Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223), 507–513 (2020).
11) Eriksen, R. S. et al. A growing microcolony can survive and support persistent propagation of virulent phages. Proc. Natl. Acad. Sci. USA 115(2), 337–342 (2018).
12) Koo, H. et al. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740–755 (2017).


