The Power of Poison

Botulinum toxins are among the most deadly substances known to man, yet have a remarkable range of therapeutic uses. David Feld, who has received injections of the toxins for 20 years, and his doctor, Richard Grünewald, explore this fascinating substance
The Biologist 65(2) p14-17
The cosmetic use of botulinum toxin, often under the trade name 'Botox', has mushroomed in recent years. However, it has a diverse and important range of clinical uses, especially in the treatment of neuromuscular disorders and chronic pain.
The story starts with a fairly typical microorganism, which most bacteriologists will never encounter and which is only ever handled in specialist laboratories. Clostridium botulinum is a typical endospore-forming, obligately anaerobic, Gram-positive rod with an optimal temperature for growth of 30–40°C.
Under appropriate anaerobic circumstances, and depending on the bacterial strain, C. botulinum can produce one of seven neurotoxic proteins, all serologically distinct and labelled as A, B, C1, C2, D, E, F and G. Type H botulinum toxin, now known to be a hybrid of types F and A, was recently recognised as being the most toxic substance known to man. Injection of a mere 2 x 10¯⁹ g is sufficient to cause death[1].
It is these toxins which produce the food-poisoning condition known as botulism. Poisoning involves ingesting the toxin through improperly preserved foodstuffs, prepared in a way that facilitates anaerobic sporulation of the organism (for example, canning or bottling). Prolonged boiling can destroy the toxins.
C. botulinum's natural target habitat appears to be the gut of animals, commonly ruminants, where the ingested clostridia produce only trivial adverse effects. The organism only occasionally causes animal botulism, usually from consumption of contaminated carcasses.
It was German physician Justinus Kerner who, in 1820, discovered botulism in a group of musicians struck down after eating contaminated ham. Kerner gave the disease the name after associating it with a similar condition caused by consumption of sausage – botulus in Latin. Much later Belgian bacteriologist Émile van Ermengem (who worked with Robert Koch) isolated the bacterium from affected attendees of a funeral.
The symptoms of botulism take more than 12 hours to develop, and include drooping eyelids (ptosis), double vision (diplopia) and dilation of the pupils (myadriasis), together with difficulty swallowing (dysphagia), dry mouth, and difficulty breathing (dyspnea) and speaking (dysphonia). These symptoms can be followed by muscle weakness. The effect on the muscle cells of the respiratory system can mean the victim is unable to take air into the lungs and eventually asphyxiates, unless respiratory support is provided[2].
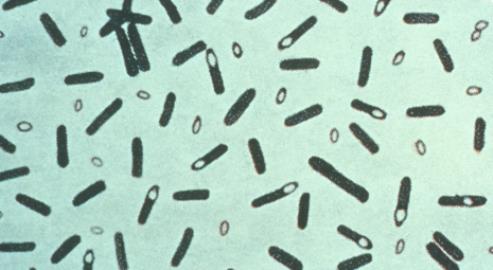 Photomicrograph of Clostridium botulinum stained with Gentian violet. From the Centers for Disease Control and Prevention's Public Health Image Library
Photomicrograph of Clostridium botulinum stained with Gentian violet. From the Centers for Disease Control and Prevention's Public Health Image LibraryBotulinum's biochemical mechanism is well understood. The toxins are not inactivated by the stomach's hydrochloric acid, and instead are hydrolysed – activated – by the bacterium's own proteolytic enzymes into two parts, which are absorbed directly through the gut wall. The smaller part is a metalloprotease, which is transported via the bloodstream and eventually binds to the terminals of cholinergic neurons – nerve cells which use the neurotransmitter acetylcholine.
From the neuronal terminals, the toxin is transported into the cell's cytoplasm. Here, it cleaves the proteins that help release the neurotransmitter – in this case, acetylcholine – resulting in that part of the nervous system being unable to secrete the neurotransmitter, and paralysis.
Harnessing harm
Botulinum toxin was introduced into clinical practice because of its property of weakening muscles. In the early 1970s, US ophthalmologist Dr Alan Scott and co-workers used minute amounts of the purified toxin to treat strabismus, or crossed eyes, in monkeys. They later used the same treatment on human strabismus[3]. It was quite contentious initially – there was fear that it would be too toxic and would kill patients, but in fact the toxin remains fairly (but not completely) localised to the injection site.
Since Scott's initial treatments, botulinum toxin has been approved in the US and the UK to weaken overactive muscles in various neurological conditions, including dystonias such as blepharospasm (inability to open the eyelids), torticollis (contortion of the neck in various planes), spasmodic dysphonia (affecting the vocal cords) and 'task-specific' disorders such as writer's cramp, musician's cramp and even 'the yips' in golfers and athletes.
The precise cause of dystonias is not known, although there are several theories, some of which might be linked in some way. The basal ganglia, a highly complex group of nuclei located at the base of the forebrain, play a key role in controlling posture and movement. In patients with dystonia, this control appears to be faulty, such that agonist and antagonist muscles co-contract and cause distortion of posture and learned movements[4].
Research at Sheffield Teaching Hospital suggests that there is a significant involvement of faulty muscle stretch receptors, known as muscle spindles, which are responsible for the perception of motion and posture[5,6]. The genetic element of dystonia is complex, and mutations in several genes have been implicated. Dystonias are also more common in Ashkenazi people, Yiddish-speaking Jews who originated in what is now called Belarus. The paternal grandfather of one of us (DRF) was Jewish and came from a small village in Poland near Lutowiska – not all that far from the Belarus border.
As dystonias are not very common, especially those of the hand-arm system[7]. they often go undiagnosed for many years.
The effect of treatment with botulinum toxin is gradual but protracted, being noticeable to the patient after four or five days. It reaches maximum efficacy after about a month and the effect wears off after about six weeks. There appears to be little or no adverse effect of long-term treatment.
As to why botulinum toxin is so effective, it has been suggested that the relative weakening of skeletal muscle, but relative preservation of muscle spindle function (the spindle is enclosed in a relatively impermeable capsule), is important. Some toxin is retrogradely transported back to the neuronal cell body and may also have an effect regionally in the spinal cord.

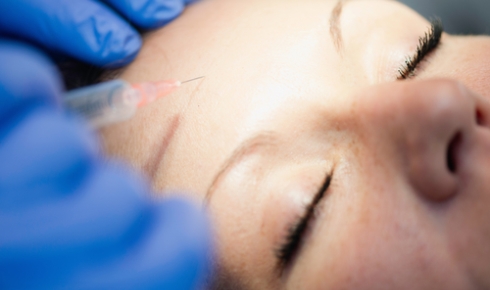
David Feld receiving injections of botulinum toxin from co-author Richard Grünewald I first came across the use of botulinum toxinin the mid-1990s at the Annual Summer Conference of the Society of Applied Bacteriology (now the Society for Applied Microbiology). There was a synchronised and suitably sharp intake of breath from the audience as a series of slides showed the eyelids of a sufferer of blepharospasm being injected with Botox. Such sufferers are registered as blind, despite their eyes working perfectly well once the eyelids are lifted.
After many years of extreme discomfort while writing, and numerous exploratory dead-ends, I was first diagnosed with focal dystonia of the hand (known as 'writer's cramp') in the mid-1990s. My first treatment with botulinum toxin was a preparation of the type A toxin, known as Dysport, and produced originally at the UK's erstwhile germ warfare establishment at Porton Down. Nearly 20 years later, the treatment continues every three months at the neurological department of Royal Hallamshire Hospital in Sheffield. The toxin is injected into muscles of the forearm that control hand movements: flexors in the fingers and thumb, and also the supinator muscle of the wrist. Doses tend to be in the 5–10 IU range for each individual injection. (A IU is defined as the dose that is toxic for 50% of poisoned mice.)
The use of botulinum toxin in the treatment of dystonias has the effect of paralysing the nerves supplying the overstimulated muscle groups, enabling 'normal' use of neighbouring groups. So, in the case of my right hand, preventing the over-rotation of the supinator allows my fingers to function more closely to normal – that is, to be able to write more comfortably.
Going mainstream
In 1989, US plastic surgeon Richard Clark used botulinum toxin to smooth out forehead wrinkles in a patient. Since then, Botox and the equivalent products of competitors (Dysport, Xeomin and Neurobloc) have been used extensively in general facial 'rejuvenation' and in other cosmetic treatments. Remarkably, the use of this deadly toxin is even becoming commonplace in spas and shopping malls. The safety is dependent on the very small quantities involved, which are only detectable by bioassay, and calls for better regulation have increased in recent years amid reports of botched procedures.
The surprisingly diverse range of uses for botulinum toxin has grown to include treatment of headaches (including migraine), squints, excessive sweating and salivation, urinary incontinence and stiff limbs after stroke. It was even described by Time magazine last year as 'the drug that's treating everything'[8]. In the US, the Food and Drug Administration agency has now ruled that the drug must carry a label which warns of the potential unpleasant side-effects of its incorrect use – such as numbness, inflammation and migration of the paralysing effect to other areas (in some cases causing drooping eyelids or difficulty swallowing).
The clinical and cosmetic uses of botulinum toxin preparations have seen a meteoric rise in less than 40 years. The very properties that make them deadly also make them valuable agents for the treatment of movement disorders, urological conditions and hypersecretory disorders.
There are now tantalising prospects for engineered botulinum toxin derivatives with altered biological target, potency and function[9,10]. Modification of the binding domain of the toxin can alter its target tissue, and if combined with alteration of the active site can be potentially converted to a drug delivery vehicle, drug activation vehicle or inhibitor of neurotransmitters other than acetylcholine[11].
Botulinum toxin derivatives have been shown to affect secretory processes related to diabetes and respiratory disorders, and processes mediating immune and inflammatory disorders. In the next 40 years, who knows what other treatments might be on offer?
David R Feld CBiol MRSB is a laboratory and quality manager with interests in mycology, conchology, taxonomy and natural history.
Richard A Grünewald is a consultant neurologist at the Department of Clinical Neurology, Royal Hallamshire Hospital.
- 'New botox super-toxin has its details censored'. New Scientist, 14 October 2013.
- WHO (2017): Botulism (reviewed, 2017).
- Scott, A. B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Journal of Pediatric Ophthalmology and Strabismus 17(1), 21–25 (1980).
- Dystonia Society (2014): The basal ganglia and dystonia.
- Grünewald, R. et al. Idiopathic focal dystonia: a disorder of muscle spindle afferent processing? Brain 120, 2179–2185 (1997).
- Grünewald, R. Dystonia and muscle spindles: the link in idiopathic focal dystonias. Mental and Behavioural Disorders and Diseases of the Nervous System, 185–198 (Intech, 2012).
- 'When my left arm let me down yet again, I finally went to the doctor'. The Times, 22 September 2012.
- 'The drug that's treating everything'. Time, p34–40, 16 January 2017.
- Kane, C. D. Novel therapeutic uses and formulations of botulinum neurotoxins: a patent review (2012–2014). Expert Opinion on Therapeutic Patents 25(6), 675–690 (2015).
- Band, P. A. et al. Recombinant derivatives of botulinum neurotoxin A engineered for trafficking studies and neuronal delivery. Protein Expr. Purif. 71(1), 62–73 (2010).
- Pickett, A. & Perrow, K. Towards new uses of botulinum toxin as a novel therapeutic tool. Toxin 3(1), 63–81 (2011)


